Living With Cystic Fibrosis
Tom Smith has never known a life without cystic fibrosis (CF), but it has not limited him or the work he does as an advocate for rare diseases. Although cystic fibrosis is a rare disease, it is one of the more common rare diseases in the UK, affecting about 1 in every 2,500 babies. Diagnosed at only six weeks old after experiencing severe breathing difficulties, Tom says that he was lucky doctors were able to identify his disease so early on so he could begin treatment.
“This was in the 1980s, when cystic fibrosis was a death sentence. The average life expectancy was only about 35 years. Things are very different today! I’m 35 years old now and expect to live a lot longer,” Tom says.
For Tom, childhood was relatively normal, and he recalls only a few instances of being hospitalized. “Until I was around 15 or 16, I felt the same as everyone else. As a teenager I became more self-conscious and wanted to fit in with my friends. I didn’t want there to be anything different about me,” Tom recalls.
Tom began putting off his daily treatments, which led to a decline in his health and more frequent visits to the hospital to stabilize his condition. Although with time, Tom’s health improved and he began prioritizing his treatments again, he still struggled with the emotional toll that can come with having CF. “Cystic fibrosis isn’t outwardly visible on most people. It was the secret that everyone knew about me, but that I never talked about. I felt like an intimate part of my identity was always being exposed,” Tom says.
Innovations in the CF Community
Over the years, the cystic fibrosis community has built up patient registries and multiple advocacy groups that have led to major advancements in treatment. “In the last 10 years, new disease modifying treatments have come on the market, including one that I have been taking because it aligns with my specific mutation of the disease,” Tom says. “It’s been incredible for me.”
For the first 2.5 years of his relationship with his wife, Tom’s morning treatments and physiotherapy appointments prevented the couple from spending a full day together. Thanks to his new medication, Tom hasn’t had to go to a physio appointment in two years and has much more energy.
“What is difficult in the CF community is that these medications don’t work for everyone. The drug I take works for most mutations but not all,” Tom explains. “There are large groups of people that are excluded and who are watching others with their disease have life-changing transformative experiences that they can’t join in on.”
In the past, Tom has applied to participate in a clinical trial, but was ineligible based on the criteria. However, he remains a major proponent of clinical research, an industry he has become very involved with, especially concerning patient engagement.
Working as a Patient Engagement Consultant
Beyond his own experience of living with a rare disease, Tom has spent much of his professional career exploring the role patients play in shaping clinical research and advancements in treatments. “Many are surprised to know that I don’t do most of my advocacy work with CF groups,” Tom says. “For my personal journey into this space, I’ve felt that only speaking and working on projects for CF is limiting in terms of my goals.”
In his early twenties, while trying to connect more with his disease, Tom found a group called Genetic Alliance UK who advocate for many rare diseases. They were looking for people to help them create materials about genomic medicine, which Tom was interested in. “That was my first experience writing plain language materials for patients before people really knew what it was.”
From there, Tom’s connections in the world of patient engagement grew exponentially. He attended a patient’s forum training session in Vienna where he was introduced to a member of The European Health Parliament. Tom applied and joined as a committee member, where he now works to shape policies that benefit patient communities.
Since then, Tom has picked up a variety of other projects, including working as a research ethics committee member for the Health Research Authority, serving as a faculty member at the European Forum of Good Clinical Practice, and working as a consultant for sponsors, medical communications companies, and regulators.
Within the clinical trials industry, informed consent and plain language are two areas where Tom enjoys taking on projects.
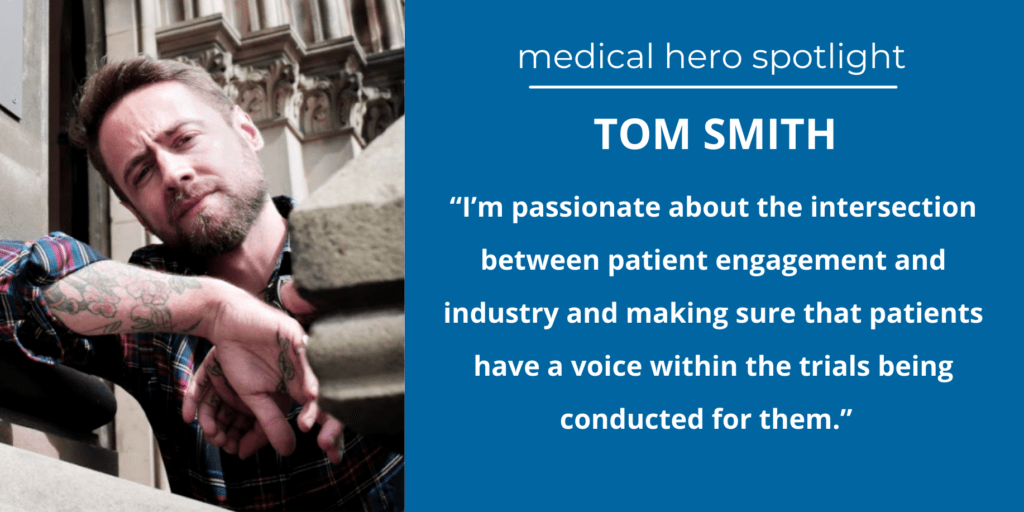
“Patients still receive documents that are 8,000 words long and full of complex medical information. They have to consent to move forward with treatment, even if the average person usually can’t fully understand what the materials say. That’s why I’m passionate about the intersection between patient engagement and industry and making sure that patients have a voice within the trials being conducted for them,” Tom says.
Encouraging Advocacy
Tom describes himself as someone who is always itching for a new challenge professionally, and he encourages other people living with chronic conditions or rare diseases to consider advocacy. Tom sees patient engagement as an “ocean beneath our feet”, with the potential to bring forth new treatments quicker, save pharmaceutical companies money, and empower patient advocates to be compensated for their work.
“We’re in the shadow of hundreds of years where doctors have controlled the outcomes for patients, when so much more could be accomplished if it were more of a partnership,” Tom explains. In his own experience, Tom has noticed that many patients settle when it comes to advocacy work because they are just excited to be involved. “If you begin advocating for yourself and your community, you can change your life! Make sure you’re being fairly compensated for your time and effort.”
All patients in a disease community are important and bring unique value. For meaningful advancements to be made, all voices need to be engaged. Tom advises patients who might be interested in getting involved in advocacy to find what interests them and start there.
“You don’t have to fit into any box as an advocate. If you’re not sure where you belong or where to start, just do what you enjoy.”
Additional Resources:
https://www.cff.org/
https://geneticalliance.org.uk/
To search for medical conditions in a specific location, visit our Search Clinical Trials page.
To stay informed about clinical trials, visit our Resources page.
For volunteer opportunities with CISCRP, visit our Volunteer page.
Written by Lindsey Elliott, Marketing & Communications Manager, CISCRP | lelliott@ciscrp.org



 In October, CISCRP’s Health Literacy team worked with a Top 25 pharmaceutical company, to create an infographic celebrating Health Literacy Month, marking the second year of this collaboration. Last year’s materials included an infographic with tips for implementing health literacy best practices and a health literacy crossword puzzle. This year’s infographic contains an exercise to help readers brush up on their health literacy knowledge and think about the importance of keeping health literacy front-of-mind.
In October, CISCRP’s Health Literacy team worked with a Top 25 pharmaceutical company, to create an infographic celebrating Health Literacy Month, marking the second year of this collaboration. Last year’s materials included an infographic with tips for implementing health literacy best practices and a health literacy crossword puzzle. This year’s infographic contains an exercise to help readers brush up on their health literacy knowledge and think about the importance of keeping health literacy front-of-mind. The Health Literacy team at CISCRP enjoyed thinking creatively about how to engage a professional audience in this health literacy exercise. One of the most enjoyable parts of the development process was incorporating movie theater imagery into the visual design to draw the audience in. We particularly enjoyed the creation of a health literacy superhero and the visual pun evoking 3D movie glasses that we used to introduce the two dimensions of health literacy. These elements are not just about having fun, but also about organizing content into digestible chunks, an important step for supporting health literacy.
The Health Literacy team at CISCRP enjoyed thinking creatively about how to engage a professional audience in this health literacy exercise. One of the most enjoyable parts of the development process was incorporating movie theater imagery into the visual design to draw the audience in. We particularly enjoyed the creation of a health literacy superhero and the visual pun evoking 3D movie glasses that we used to introduce the two dimensions of health literacy. These elements are not just about having fun, but also about organizing content into digestible chunks, an important step for supporting health literacy.





































































